
Morphology
of the Life Stages of three Heterorhabiditis spp. from the Infective
Juvenile to the Hermaphrodite
Khuong B. Nguyen and Grover C. Smart, Jr.
Entomology & Nematology Department
University of Florida
Soil and Crop Science
Society of Florida Proceedings 1998 - Volume 57:101-107
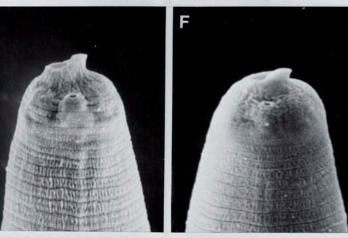
Infective juvenile head of H. megidis and H. bacteriophora
ABSTRACT
Three species of Heterorhabditis
were studied using scanning electron microscopy. In all three species,
the sheath of the infective stage juvenile (the sheath is the cuticle of
the second-stage) is tessellate anteriorly and bears longitudinal ridges
throughout almost the entire body length. The infective juvenile, and the
third stage which develops from it, have a dorsal tooth on the labial region.
The fourth stage has low lips
and labial papillae, and a rounded oral aperture. The hermaphrodite has
massive lips with prominent labial papillae at the apex of the lips and
a hexagonal mouth cavity.
The family Heterorhabditidae contains only
one genus, Heterorhabditis, described by Poinar in 1975 (4). Currently,
eight species have been described. The morphological characteristics of
these species are similar, and they have been differentiated mostly based
on morphometrics of the IJ (third-stage infective juvenile). Few studies
have been done on the morphology of other juvenile stages or adults of
this group of nematodes. Mracek et al. (1) published scanning electron
microscopy (SEM) photographs of the anterior part of a J2 (second-stage
juvenile), the labial region of a female (although the authors did not
mention the type of female, we think it was the hermaphrodite), the excretory
pore, and the tail of a female (probably the hermaphrodite). In a second
paper, Mracek and Bednarek (2) reported on cuticular structures of the
J2 and the IJ of Heterorhabditis spp.
In this paper, we report our observations
on the morphology of life stages from the infective stage juvenile to the
hermaphrodite for three species of Heterorhabditis.
MATERIALS AND
METHODS
The three species studied were Heterorhabditisbacteriophora
Poinar, 1975 from our collection; H. hawaiiensis Gardner
et al., 1994; and H. megidis Poinar et al., 1987 from Dr.
S. P. Stock, Dept. Nematology, Univ. of California, Davis. All three species
were maintained in our laboratory by producing them on larvae of the greater
wax moth, Galleria mellonella (L.) at 25o C. Ten
G.
mellonella
were exposed to about 10,000 IJs of each species in a petri dish (100 x
15 mm) containing a filter paper (90 mm). Each day after exposure until
new IJs emerged, one insect cadaver infected with each nematode species
was washed 3 times in tap water to remove any IJs on the exterior of the
insect. Then the insect was dissected in a saline solution (1% NaCl in
water) to collect nematodes. The nematodes were washed three times with
saline solution, and then prepared for SEM observation by the method of
Nguyen and Smart (3).
RESULTS AND DISCUSSION
Third-stage
infective juvenile (IJ) before entering host(Fig.
1) : The IJ initially is encased in the
cuticle of the second-stage juvenile (J2). The J2 cuticle is usually lost
after storage or after entering the hemocoel of insects. The cuticle of
the J2 of all three species has longitudinal ridges throughout most of
the body length, and a tessellate pattern in the most anterior part of
the body (Fig. 1A-C). The IJ of H. megidis
has a prominent dorsal tooth associated with a distinct membranous ring
around the oral aperture (Fig.1 D, E).
The IJs of H. bacteriophora and H. hawaiiensis
have a dorsal tooth, but the membranous ring is inconspicuous (Fig. 1F).
The purpose of the membranous ring is not known, but it may play a role
in penetrating the intestinal wall of an insect host. The two small subventral
teeth described by Poinar and Georgis (5) were not observed in any of the
three species studied. Amphids were circular and located on a protuberance
(Fig.
1D-F). Neither lips, nor labial or cephalic papillae, were observed.
The body was annulate. The lateral field began with one incisure anteriorly
(Fig.1D), followed by three incisures posteriorly resulting in two ridges
(Fig. 1G-I). The middle incisure appears deeper and wider than the two
lateral ones. Except for the presence of the membranous ring in the labial
region of
H. megidis only, no other differences were found
in the general morphology of the IJs for H. bacteriophora,
H.
hawaiiensis, and H. megidis.
Day one after
entering host: After entering the insect body cavity, the
IJ changed to a feeding third-stage juvenile (J3) and the body enlarged.
The base of the dorsal tooth also enlarged and thickened to become well-sclerotized
anteriorly (Fig. 2A-C).
A few individuals of H. hawaiiensis began the molting process.
Day two after
entering host: The J3 increased in size, and if the molting
process had not begun at the end of day one, it began during day two. The
cuticle loosened anteriorly, causing the labial region to collapse when
specimens were prepared for SEM (Fig.
2D); the cause of labial collapse can be seen by light microscopy as
the clear space between the two cuticles (Fig. 2E). Under the light microscope,
a solid structure, in addition to the dorsal tooth, is present (Fig.2E).
Since this structure does not appear in SEM micrographs it must be subcuticular.
The J3 of H. bacteriophora and H. megidis had
the same labial appearance (Fig. 2D), indicating similar growth rates to
this point. H. hawaiiensis developed faster than the other
two species, with most of the J3s molting to fourth-stage juveniles (J4s)
(Fig. 2F). The labial region of the H. hawaiiensis J4 was
similar to that of H. bacteriophora, as described below.
Day three after
entering host (Fig.
3): Most of the J3s of H. bacteriophora molted to J4s
by day three (Fig. 3A-C). The dorsal tooth was not present in the J4, and
the cheilorhabdions appeared as a ring in the mouth. There were six lips,
each with one indistinct papilla situated at a distance from the oral aperture;
the amphids were less conspicuous than in the IJ and J3.
For H. megidis, both J4s
and young hermaphrodites were present. The labial region of the J4 (Fig.
3D) was similar to that of H. bacteriophora (Fig. 3 A-C).
The hermaphrodites of H. megidis (Fig. 3E, F) had prominent amphids,
lips, and labial papillae. The lips were at the edge of or just in side
the mouth cavity giving the oral aperture a hexagonal shape (Fig. 3E,F).
By day 3, all juveniles of H. hawaiiensis had become young
hermaphrodites with prominent lips and labial papillae, similar in shape
and size to those of H. megidis but curved outward (Fig. 3G-I) .
Days four and
five after entering host (Figs.
4): Four days after entry, most of the juveniles of all three species
had become young hermaphrodites; by day 5 they became mature adults. The
lips were large and elevated, sometimes curved outward, each with a labial
papilla at its apex (Fig. 4 A-I). The ten cephalic papillae described by
Poinar et al. (6) for H. megidis, if present, were not obvious
on any of the three species that we studied. Sometimes one or two small
dots were observed at the base of each lip, but they were not prominent
in our preparations (Figs. 4).
The vulval area (Fig.
5 A-C) of the three species of Heterorhabditis is reminiscent
of the perineal pattern of Meloidogyne spp. (7). The vulva of H.
bacteriophora
(Fig.5A) is characterized by an elliptical opening with rings around it
which extend into the vagina. In H. megidis (Fig. 5B), the
posterior vulval lip is depressed, the annules extend into the vagina,
and the annules anterior and posterior to the vulva form acute angles at
the lateral margins of the vulva. The vulva of H. hawaiiensis
(Fig. 5C) is slit-like, the vulval lips are prominent, the annules do not
extend into the vagina, and the annules around the vulva are wavy or broken.
The tail of the hermaphrodite is conoid (Fig. 5D), and the anus, which
appears to be curved, is on a protuberance.
CONCLUSIONS
SEM observations of three species of Heterorhabditis
suggest several conclusions.
Based on morphological characters of the
labial region, the rate of development of H. hawaiiensis is greater
than that of H. megidis and H. bacteriophora.
The growth rate between the three species
was different by days two and three after infection. For example, by day
two after infection, most of the H. hawaiiensis juveniles
had become J4s (Fig. 2F)
whereas those of H.
bacteriophora and H. megidis
remained as J3s (Fig. 2A-E). By day three, most of the H. bacteriophora
juveniles had become J4 (Fig.
3A-C), whereas H. megidis had a mixed population of J4
and young hermaphroditic females (Fig. 3D-F), and all juveniles of H.
hawaiiensis
had become young hermaphroditic females (Fig. 3G-I).
For all three species, the morphology of
the labial region and the cuticle of the J2, IJ, J3, J4 and the adults
are different. The oral aperture of the J2 is almost closed, the cuticle
is tessellate anteriorly, and longitudinal ridges occur over almost the
entire body posteriorly. The IJ, and the J3 which develops from it, have
a dorsal tooth in the labial region. The body has annules and lateral fields
with two ridges. Longitudinal ridges present in the J2 are absent. The
J4 has low lips and labial papillae, and a rounded oral aperture. The lips
of the hermaphrodite are massive with prominent, anteriorly directed labial
papillae at their apex. The arrangement of the lips creates a hexagonal-shaped
mouth cavity. All of the above characters of the different stages are consistent
for the three species studied.
The structure of the vulval region of the
three species seems different, and may or may not be different enough to
use for species identification.
LITERATURE CITED
1. Mracek, Z., J. Weiser, and E. Arteaga
1984. Scanning electron microscope study of Heterorhabditis heliothidis
(Nematoda: Heterorhabditidae). Nematologica 30:112- 114.
2. Mracek, Z., and A. Bednarek. 1992. Cuticular
structures of J2 and J3 of Heterorhabditis. Nematologica 38:386-390.
3. Nguyen, K. B., and G. C. Smart, Jr.
1995. Scanning electron microscope studies of Steinernema glaseri
(Nematoda: Steinernematidae). Nematologica 41:183-190.
4. Poinar, G. O., Jr. 1975. Description
and biology of a new insect parasitic rhabditoid, Heterorhabditisbacteriophora
n. gen. n. sp. (Rhabditida; Heterorhabditidae n. fam.). Nematologica 21:463-470.
5. Poinar, G. O., Jr., and R. Georgis.
1990. Charactization and field application of Heterorhabditis bacteriophora
strain HP 88 (Heterorhabditidae: Rhabditida).
Revue de Nematologie 13:387-5
6. Poinar, G. O., Jr., T. Jackson, and
M. Klein. 1987. Heterorhabditis megidis sp. n. (Heterorhabditidae:
Rhabditida) parasitic in the Japanese beetle, Popillia japonica
(Scarabaeidae: Coleoptera), in Ohio. Proc. Helminthological Soc. Washington
54:53-59.
7. Taylor, A. L., and J. N. Sasser. 1980.
Biology, identification and control of root-knot nematodes (Meloidogyne
species). North Carolina State Univ. Graphics, Raleigh, NC.
Updated on 6 October, 2012
Entomology & Nematology Department
University of Florida
All constructive comments are welcome, please
Email
to:kbn@ufl.edu

